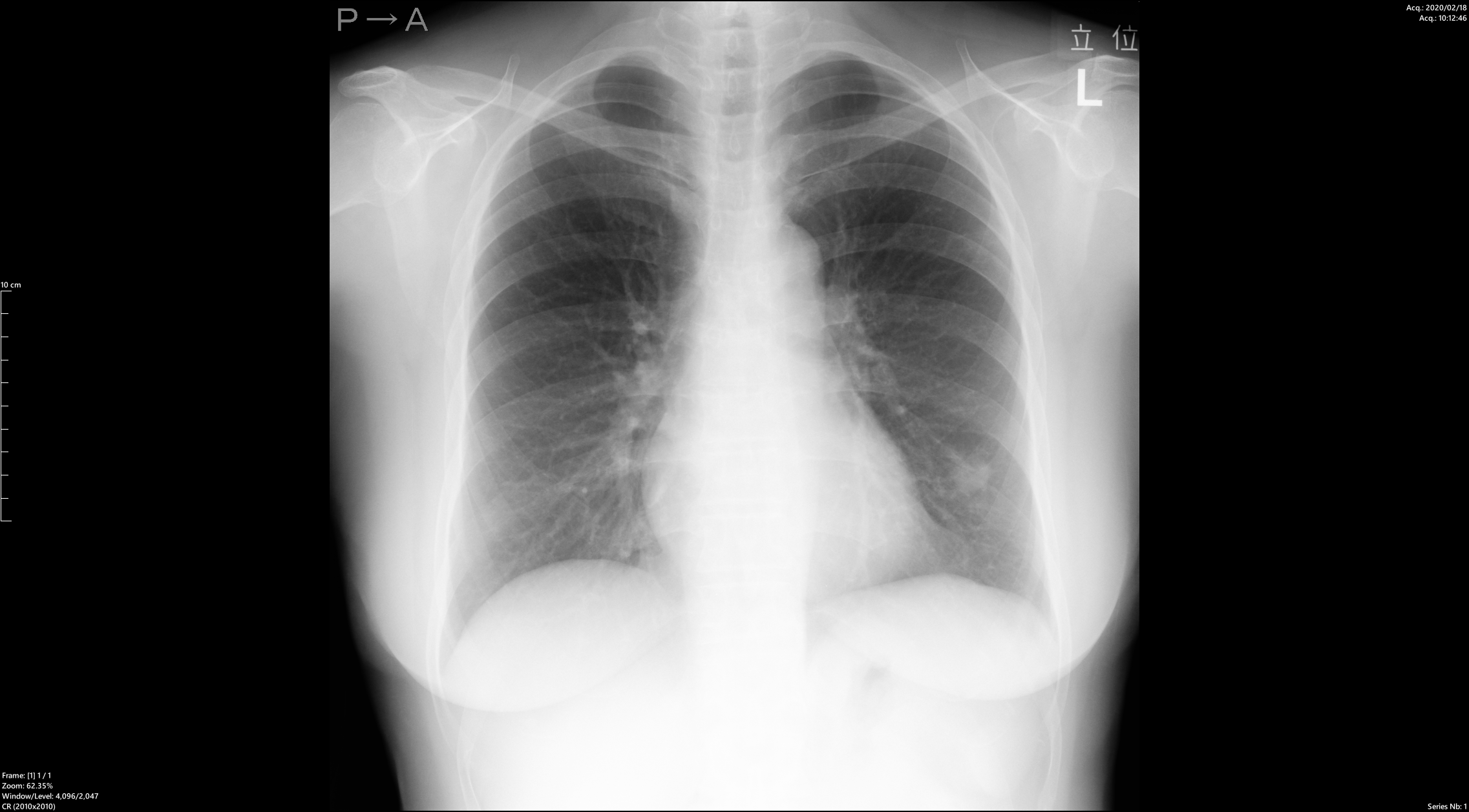
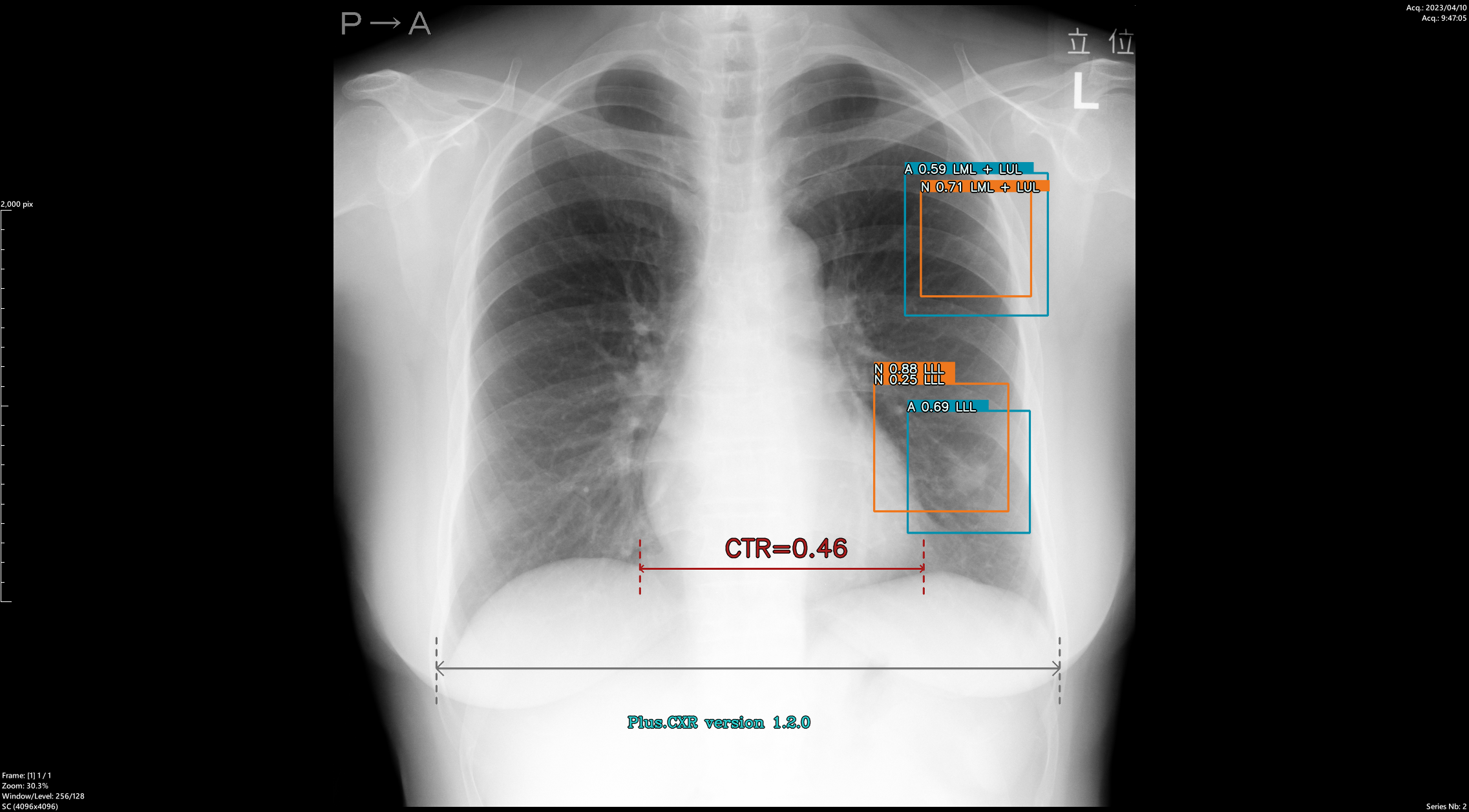
Dr. Murayama: In terms of interpretation time, compared to before, I now spend a bit more time looking at the images carefully. That’s because in the past, I didn’t consciously pay attention to 1–2 mm nodules—I may have thought of them all as parts of blood vessels.But after introducing PLN, I realized that almost everyone has nodules around 2 mm.(※)
Years ago, Dr. Swensen published a paper reporting that about 70% of people had nodules in chest CT screenings. I assumed that was a characteristic of American patients and that fewer Japanese people had nodules. However, after using PLN, I found that most people had nodules ≥2 mm, confirming that what Dr. Swensen said was indeed correct. Now I think it’s natural to assume that almost every patient has nodules of at least 2 mm.
There are intrapulmonary lymph nodes near the pleura, and it turns out such small nodules exist in most people. I struggled at first with how to interpret them, but I now write in reports: “Several small nodules are observed, but they are not considered clinically significant.” For round nodules of ≥3 mm or nodules of ≥5 mm, I pay close attention. Nodules under 3 mm are rarely malignant, but for ≥5 mm, the risk increases, and I make sure to write a detailed report since they could represent early-stage cancer.
So yes, I now understand that there are many even smaller nodules—but I didn’t realize that before using PLN. It truly triggered a paradigm shift in my thinking.